PHASE Lab HPV Urine Test

- First of Its Kind
- Patented PHASiFY Technology
- Non-Invasive Cervical Cancer Screening
- Comprehensive High-Risk HPV Detection
- Clinic and At-Home Options
About
Our test offers high-quality DNA-based detection of 14 high-risk HPV subtypes using a non-invasive urine sample. Our advanced liquid phase extraction technique, PHASiFY, ensures exceptional accuracy. Provide your patients with top-tier HPV testing and unmatched convenience.
Benefits of Urine Testing
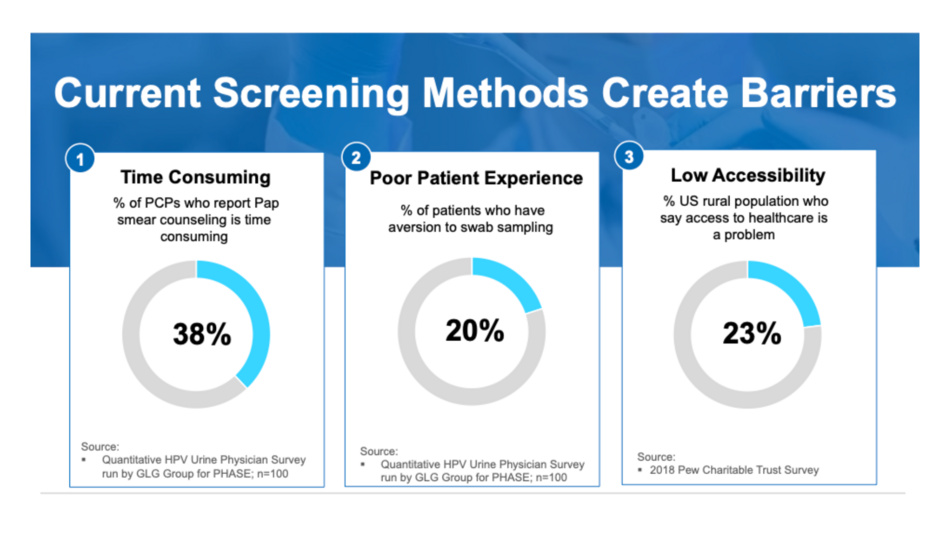
- Utilizes PHASiFY technology for high recovery of viral DNA which allows our test to achieve the high specificity and sensitivity typically found in cervical swab sampling.
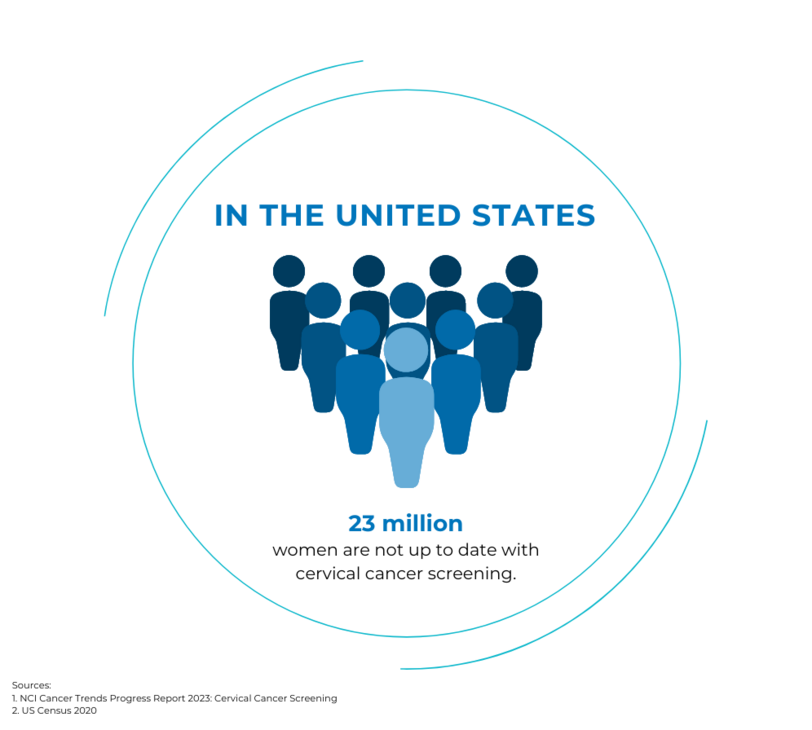
- Removes barriers patients may have around physical, psychological, social, and cultural factors that can hinder HPV testing. Urine is a non-invasive and familiar option to most patients.
- Saves time in counseling patients who are averse to cervical swab sampling.
- Removes the need to refer to a specialist or alternate provider for cervical exam and HPV screening.
Specifications
| Intended Use | The PHASE Lab HPV Urine Test is a qualitative in vitro real time PCR test for the detection of Human Papillomavirus (HPV) in first catch urine specimens self-collected at home or at a healthcare setting from women 18 years or older. |
|---|---|
| Results Reporting | The test provides differentiated results for HPV 16 and HPV 18 and grouped results for the other 12 high risk subtypes (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). |
| Sample Type | Urine (first catch, any time of day) |
| Sampling Method | Urine collection kit provided for use in a healthcare setting or at home |
| Assay Method | DNA detection by PCR with PHASiFY technology |
| Certifications | Samples tested at the CLIA/CAP-accredited PHASE Lab |
| Turnaround Time | 3 business days after receipt of the sample in lab |
Frequently Asked Questions
A small sample of urine will be self-collected at the provider's office in the cup provided. The provider's office will pack and ship the sample to PHASE Lab for testing. PHASE Lab will perform the testing utilizing PHASiFY technology. The results will be reviewed by an expert and released to the patient and the doctor.
Our target performance is to be comparable to FDA-cleared HPV tests using clinician collected cervical brush samples. Preliminary research indicates that our novel HPV urine test may perform as well as, or even better than, traditional Pap smears.
The process takes about 2-3 days from when the urine sample is received in our laboratory.
Results will be sent to the doctor and patient through the INDICAID health portal.
Contact Sales & Customer Service

Customer Service

