Easy Lab PRO Rapid RSV Test (Point-of-Care)
FDA-cleared and CLIA-waived testing for the detection of RSV antigens
- FDA cleared and CLIA waived
- For Prescription Use Only
About
PHASE Scientific Americas, in partnership with VerséaTM, is now the Master Distributor of the Easy Lab PRO Rapid RSV Test. We are excited to add an RSV test to our ever-expanding portfolio of PoC offerings, especially given the rising prevalence of RSV over the past few years. The Verséa Easy Lab PRO RSV Rapid Antigen Test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab and nasal aspirate specimens in children less than 6 years and adults over the age of 60.
Features
- Quick: Users can expect quick results in just 15 minutes.
- Easy: Features an easy-to-follow four-step testing procedure.
- Simple Sampling: User-friendly, multi-sample convenience using nasopharyngeal swab and nasal aspirate specimens
- For Pediatrics and Adults Over 60 : Intended for high-risk patients, children less than 6 years old, adults over the age of 60, and patients with symptoms consistent with RSV infection
Specifications
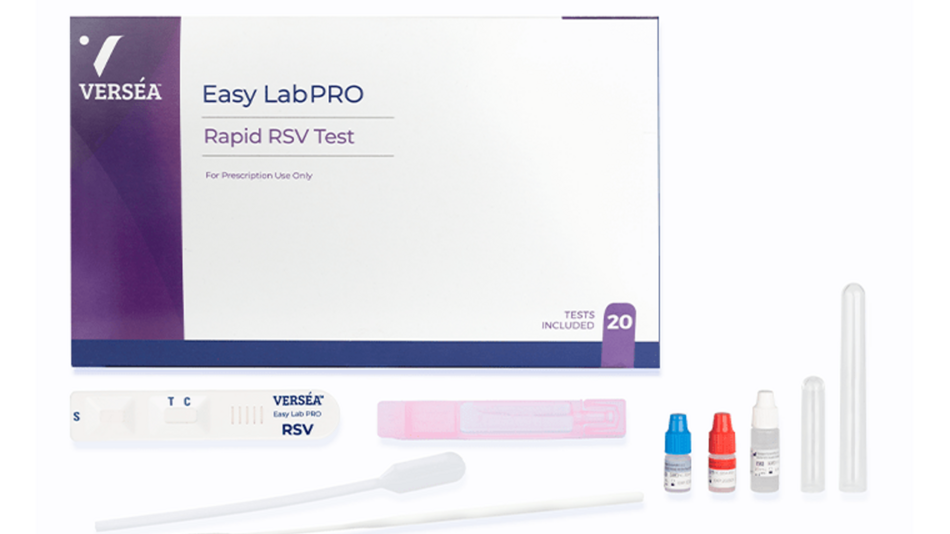
| Test Components | 20 Test Devices (Individually Packaged), 20 Sterile Nasopharyngeal Swabs, 20 Specimen Processing Tubes, 20 Sterile Normal Saline Vials (Individually Packaged), 20 Extraction Tubes, 40 Disposable Pipettes, 1 R1 (Extraction Reagent Solution), 1 PC (Positive Control Reagent), 1 NC (Negative Control Reagent), 1 Instructions for Use |
|---|---|
| Items Per Box | 20 |
| Specimen Type | Nasopharyngeal Swab and Nasal Aspirate Specimens |
| Storage Condition | 35°F to 86°F (2°C to 30°C) |
| Shelf Life | 12 Months from Date of Manufacture |
| Accuracy | Sensitivity: 93%, Specificity: 97% |
Intended Use
The Verséa Easy Lab PRO RSV Rapid Antigen Test is intended for the rapid, qualitative detection of respiratory syncytial virus fusion protein directly from nasopharyngeal swab and nasal aspirate specimens in children less than 6 years and adults over the age of 60. The test is intended for use as an aid in the rapid laboratory diagnosis of acute respiratory syncytial virus infection in patients with symptoms consistent with RSV infection. It is recommended that negative test results be confirmed by cell culture.
Contact Sales & Customer Service

Customer Service

